Ten Steps For Identifying Personalized Guide For CBD Oil For Depression (With Pictures)
In case of a positive test, the subject was excluded from the study. Subjects were instructed not to eat for at least 6 hours and drink for at least 2 hours before the study visit.
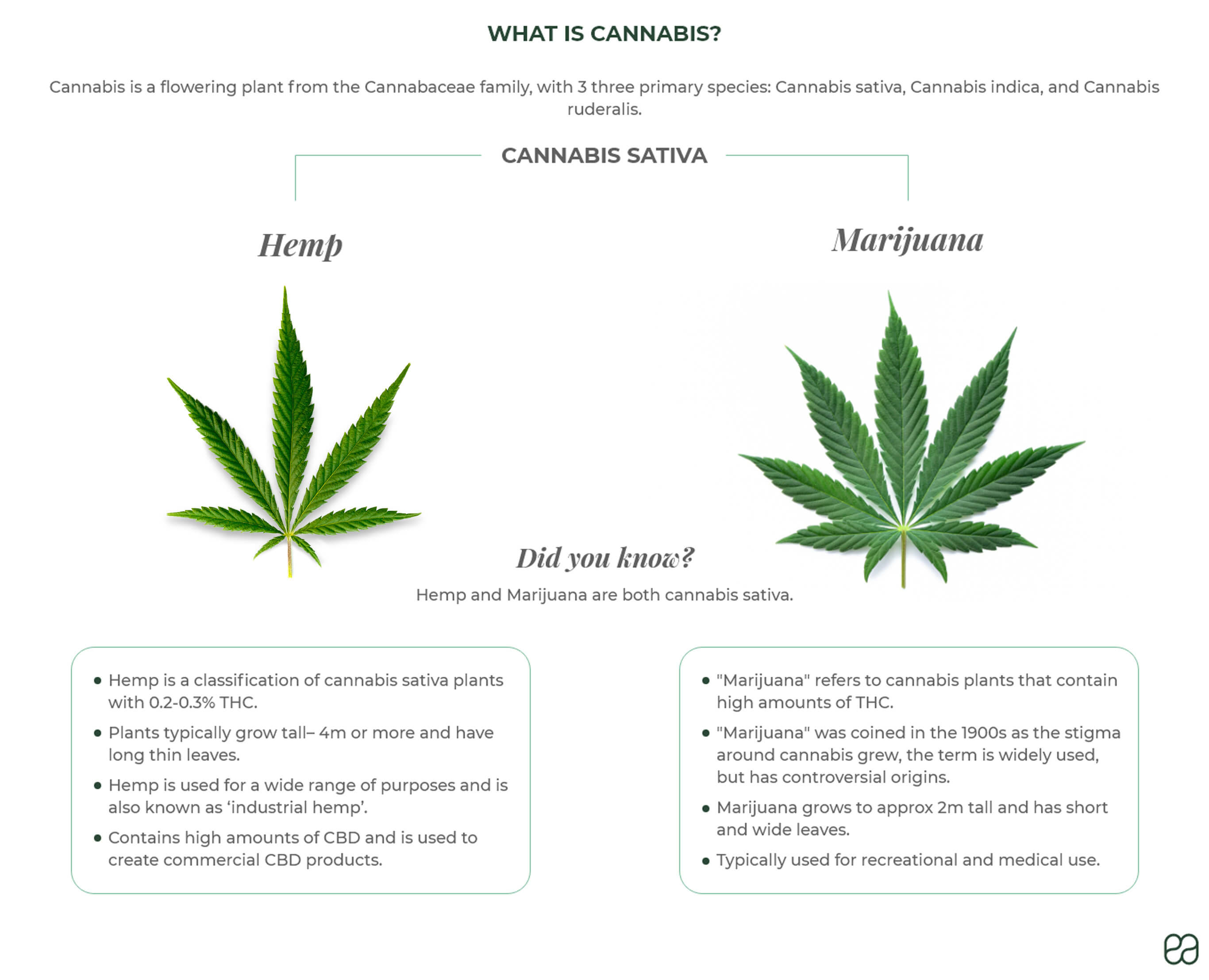
Medical Cannabis A Potential Money
We also must be mindful of “gimmick” products for which there is no reason to believe CBD is a meaningful addition. A revealing epidemiological study last year identified a distinct association between increasing THC levels in common cannabis strains and higher incidences of psychotic disorders. This would appear to be good news for Ms. Smith and Mr. Jones but there are still many other issues that need to be considered. Using CBD from hemp still can be regulated by the FDA – Mr. Smith and Ms. Jones need to comply with the FDA regulations. Additionally, the Farm bill only legalizes the product of CBD based hemp products at the federal level.
Any foods or beverages containing caffeine such as coffee, tea, or chocolate were not allowed for 24 hours before the study visit. A study has shown that cannabidiol slows cancer cell growth and is toxic to human glioblastoma cell lines, presenting a possible treatment. Cannabidiol might decrease how quickly the liver breaks down some medications. In theory, using cannabidiol along with some medications that are broken down by the liver might increase the effects and side effects of some medications.
Packaging of extracts and syringes must comply with FDA labeling requirements. When a CBD extract is used as a standalone product it is often placed under the tongue “via sublingual application” or it is swallowed. , pregnancy, breast feeding, and the presence of pain syndromes other than FM.
- Researchers focused first on these animals because their condition closely mimics the characteristics of human arthritis, the leading cause of pain and disability in the U.S. for which there is no effective treatment.
- Drug Enforcement Administration, and obtain approval from the National Institute on Drug Abuse.
- Given the paucity of data in this area, clinical observations can be quite useful to advance the knowledge base and to offer questions for further investigation.
- To conduct clinical drug research with Cannabis in the United States, researchers must file an Investigational New Drug application with the FDA, obtain a Schedule I license from the U.S.
- The present study describes a series of patients using CBD for treatment of anxiety or sleep disturbances in a clinical practice setting.
Those calculations were a key part of the study, Dr Koturbash told NutraIngredients-USA. Almost all of the dosage/safety information that exists about CBD comes from the work done by English company GW Pharma in the development of its drug Epidiolex. The drug was approved last year by the US Food and Drug Administration for the treatment of seizures in two fairly rare forms of childhood epilepsy. A study published recently has found a level of liver toxicity for CBD, putting up a big caveat to the notion within the hemp/CBD world that its products have nothing in the way of safety concerns.
Before using cannabidiol, talk to your healthcare provider if you take any medications that are changed by the liver. Analysis in the report covers such issues as allowable THC levels allowed in European hemp, CBD in health and beauty products, market indicators, key trends and developments, the effect of foreign competition and transparency issues.
Her research focuses on the variability of drug concentrations and response in topics related to the elderly, pregnant women, children, and drug addiction. Dr. Birnbaum’s research includes investigation of epilepsy medications including medical cannabis. Patients often experience mood elevation after exposure to Cannabis, depending on their previous experience.
More In Medicine & Health
In order for Smith and Jones to begin their California business, they need to know whether their hemp-based CBD edibles, lotions, and other products are legal in California. Additionally, Smith and Jones need to review whether the Drug Enforcement Agency approves hemp. CBD extract can be sold as a standalone product or as an ingredient in other products. When sold as a standalone, a syringe or similar product is usually included. Extracts are oily substances that contain cannabinoids, terpenoids, and fatty acids.
